The Role of Caloric Restriction and Glutamine Targeting in the Press-Pulse Therapeutic Strategy
Introduction
The management of metastatic cancer presents significant challenges, particularly due to the metabolic diversity of tumors. While some tumors primarily utilize glucose for energy, others are heavily reliant on glutamine. The Press-Pulse therapeutic strategy, as outlined by Seyfried et al. (2017), offers a comprehensive approach to cancer treatment by targeting these metabolic dependencies. This article explores the implications of combining caloric restriction with glutamine targeting DON (6-diazo-5-oxo-L-norleucine) and the use of 2-deoxyglucose (2-DG) to enhance therapeutic outcomes in metastatic cancer.
Understanding Tumor Metabolism: Glucose vs. Glutamine
Tumors exhibit varying metabolic profiles, with some relying predominantly on glucose and others on glutamine. Glutamine-dependent tumors, such as the VM-M3 glioblastoma model, are often less detectable using standard imaging techniques like FDG-PET but can be identified through glutamine-based PET imaging. This distinction underscores the importance of developing targeted therapies that address the specific metabolic needs of different tumor types.
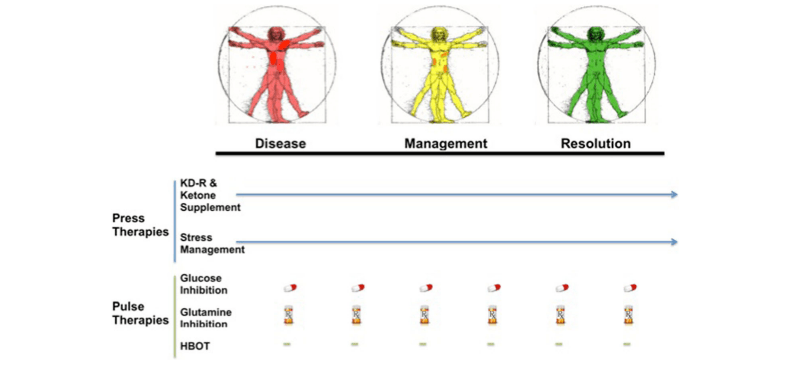
Caloric Restriction as a Press Disturbance
Caloric restriction (CR) serves as a chronic metabolic stressor, reducing systemic glucose availability and elevating ketone body levels. While CR has shown promise in reducing primary tumor growth and limiting distal invasion in models like VM-M3, it does not completely prevent systemic metastasis. This limitation highlights the need for additional therapeutic strategies to effectively target glutamine-dependent tumors.
Glutamine Targeting: A Pulse Disturbance
Glutamine targeting involves the use of specific inhibitors to disrupt the availability of glutamine, a critical fuel for many tumors. The glutaminase inhibitor DON (6-diazo-5-oxo-L-norleucine) has demonstrated therapeutic benefits in clinical settings, particularly when combined with glycolysis inhibitors like lonidamine. Other glutamine inhibitors, such as BPTES and CB-839, also show potential in targeting glutamine-dependent tumors.
However, careful consideration of the potential adverse effects of glutamine targeting is essential, as glutamine plays a vital role in various physiological functions, particularly in immune cell metabolism. Periodic glutamine supplementation may be necessary to maintain immune function while maximizing therapeutic benefits.
The Role of 2-Deoxyglucose (2-DG)
2-Deoxyglucose (2-DG) is a glycolysis inhibitor that has shown promise in the metabolic management of cancer. In preclinical studies, 2-DG has been found to have no therapeutic effect on tumor growth when administered alone to mice on a standard high-carbohydrate diet. However, when combined with a caloric restriction or a restricted ketogenic diet (KD-R), 2-DG exhibited a powerful therapeutic effect against tumors, such as the CT-2A malignant glioma model.
This synergistic interaction suggests that the KD-R creates a chronic energy deficit, making tumor cells more susceptible to the acute effects of 2-DG. The combination of CR or KD-R as the press disturbance and 2-DG as the pulse disturbance exemplifies the potential of the Press-Pulse strategy to enhance treatment efficacy while minimizing toxicity.
Synergistic Effects of Caloric Restriction, Glutamine Targeting, and 2-DG
The combination of caloric restriction, glutamine targeting, and 2-DG has shown synergistic effects in preclinical studies. While CR alone can reduce tumor growth, the addition of DON significantly enhances the reduction of both primary tumor size and systemic metastasis. Furthermore, the incorporation of 2-DG into this regimen can further amplify the therapeutic effects, as it targets the glycolytic pathway that tumor cells rely on for energy.
In this context, CR acts as the press disturbance, creating a chronic energy deficit, while DON and 2-DG serve as pulse disturbances, delivering acute metabolic stress to glutamine-dependent and glucose-dependent tumor cells, respectively.
Optimizing the Press-Pulse Strategy
The success of the Press-Pulse therapeutic strategy hinges on the optimization of scheduling, timing, and dosing of the various interventions. This includes determining the order of pulse delivery, the duration of press and pulse therapies, and the appropriate dosages of drugs to minimize toxicity while maximizing tumor cell death. Individual patient factors, such as age, sex, and overall health, should also be considered in tailoring the treatment approach.
Conclusion
The Press-Pulse therapeutic strategy represents a promising framework for the metabolic management of metastatic cancer. By integrating caloric restriction with targeted glutamine inhibition and the use of 2-DG, this approach aims to exploit the unique metabolic vulnerabilities of tumors while preserving normal cell function. As research continues to advance, the optimization of this strategy may lead to improved therapeutic outcomes and enhanced quality of life for cancer patients.
For further insights, refer to the original article: Press-Pulse: A Novel Therapeutic Strategy for the Metabolic Management of Cancer.